Introduction
When it comes to the search for extra-terrestrial life, silicon is a fan favourite among scientists and science fiction alike as the most likely element besides (or after) carbon to act as a chemical backbone for life. Silicon and carbon share many similar properties which might suggest the two are practically interchangeable, so what makes them so different to one another after all? Why didn’t life choose silicon in the first place, nor incorporate much along the tree of life? Of course, we cannot answer these questions with certainty considering we have not found life outside of our planet, but we have enough reason to investigate. For the rich variety of planets we have in our universe, it’s unlikely we’ll find carbon-based organisms just like ourselves once more – so perhaps silicon seems the right alternative.
Chemical Properties
Both silicon and carbon are found in group four, with silicon placed just below carbon in the group with 10 more electrons (Fig. 1), which means the electrons on a silicon atom’s outer shell are found on a higher energy level. Due to this, the outermost electrons in a silicon atom are further away from the nucleus, which gives silicon a slightly larger atomic radii than carbon, and in principle suggests silicon is more reactive than carbon because such outer electrons are thus less strongly attracted by electrostatic forces of the positive nucleus and should be more easily lost in reactions. 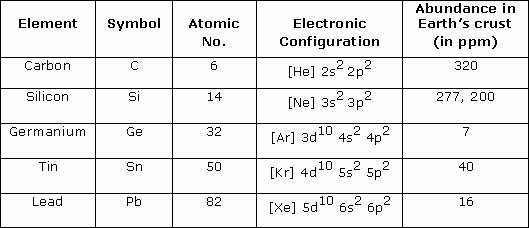
Figure 1
Therefore, we could postulate that silicon would stir the pot in the primordial soup of some alternate planet and form complex biological molecules quickly and with ease. Ironically however, silicon’s tendency to react so readily acts as double-edged sword as it means silicon is never found in its pure form on Earth; instead ‘trapped’ in generally inert compounds such as silicon dioxide, which constitutes to 27.7% of the Earth’s crust (Britannica, 2024). Also, silicon is only so reactive at higher temperatures, so if we were to find silicon on an Earth-like planet (a planet with similar conditions to Earth), silicon-based compounds would not be the biosignatures we would be looking for. Perhaps then, this property would be beneficial on hotter planets where silicon and its compounds could be found in a variety of states, but finding a substance that could remain a liquid at such high temperatures to transport substances through our hypothetical silicon organism is a very difficult task.
Onto bonding, carbon is significantly more electronegative than silicon (Fig. 2), and is thus able to form hydrogen bonds between other molecules, which is the strongest intermolecular force. Silicon, on the other hand, as it is almost always found as an oxide, is incapable of forming any intermolecular forces as silica (silicon dioxide) is a macromolecular structure. This is significant as hydrogen bonding in carbon compounds, notably within proteins, dictates a protein’s unique shape and structure (both secondary and tertiary). It’s also worth taking into account that it is theorised (and widely accepted) that amino acids were the first biomolecules to come about, so in the absence of amino acid-like substances at the very least, could life even in its simplest form come about?
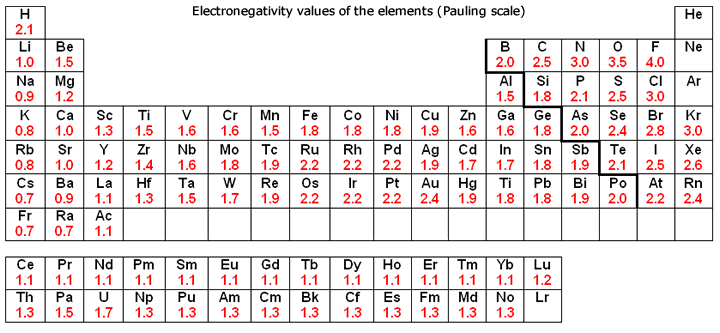
Figure 2
Given both silicon and carbon are in group four, both elements can form four bonds per atom, and both elements usually react with such to form covalent compounds (Petkowsk, Seager, & Bains, 2020) However, a major difference between the two are their tendencies to react with different elements. As explained before, silicon itself is more reactive than carbon, but if we are to consider the thermodynamics, carbon is more reactive than silicon because it requires less energy to react with other elements, so such reactions are more likely to take place than silicon ones. The major ramifications of this are if we were to find a planet and add silicon and carbon to it, carbon-based compounds are simply more likely to arise. If we were to find an exoplanet planet with either silicon or carbon as well as oxygen (in both instances), silicon is very likely to be found as silica due to its high oxygen affinity whereas carbon is could to be found as carbon dioxide, carbon monoxide, carbon trioxide etc. suggesting silicon-based life is improbable given a silicon-based organism would be unlikely to react with and thus alter the environment it’s in which at the very least prevent any sort of biochemical processes from occurring, and on a larger scale would prevent such an organism from affecting the planet it is living on; not allowing for the evolution of more complex and intelligent life, as well as a variety of life because such an inert planet would not be conducive for evolution as organisms would not need to adapt to any new challenges.
Physical Properties
In terms of atomic mass, silicon is significantly heavier than carbon given it has an atomic mass of 28 whereas carbon’s is only 12. This means compounds based of silicon would generally have higher melting and boiling points than their carbon counterparts, which, as stated before, might not be a problem on hotter planets, but it does pose a challenge when we come to consider the physiology of an organism that would survive and undergo internal synthesis and decomposition reactions at a high enough body temperature, which wouldn’t interfere with the bonds related to the organism’s internal structures such as its ‘skin’ or organs or anything similar.
Furthermore, if we are to consider the water paradox, how could an equilibrium between these synthesis and degradation reactions be maintained for the formation of more complex molecules if the temperature favours the former? Perhaps this could be rectified if such a hot planet had seasons, but how long or severe these seasons would need to be is very difficult to predict. Moving on from the dry, back to an Earth-like planet, the water paradox argues that though water was needed for the first biomolecules to come about. ‘Dry phases’ where water was removed were also needed for substances to come together in a primordial soup ( (Morato, 2022)). Essentially, on an exoplanet with similar environmental conditions to Earth then, silicon and its compounds would be incapable of being easily recycled and repurposed for any future generations of silicon organisms as silicon and its compounds are not water-soluble.
Silicon-Based Biosphere
In this theoretical Silicon Valley we have created, what conditions would be needed for silicon-based life to function remotely similar to life as we know it? While water is not a suitable solvent for silicon-based life (as it could only exist as a gas while silicon is solid), an aprotic solvent could be suitable given they can dissolve a wide range of elements (Petkowsk, Seager, & Bains, 2020), however, given they are usually only liquids at very low temperatures, this itself limits the possibility of reactions, so this probably won’t be the case. Cell membranes could likely not be based off silicon due to silicon’s inability to form long chains; preventing the formation of bilayers with polar and non-polar components that would allow for the controlled movement of substances. Furthermore, given silicone gel, a common desiccant, can absorb water and release water in continuous cycles (Wikipedia, 2023), could this if present on some faraway exoplanet, allow for the wet and dry phases needed if this silicone gel could act as some sort of cytoplasm?
However, given silicone gel, a common desiccant, can absorb water and release water in continuous cycles (Wikipedia, 2023), could this if present on some faraway exoplanet, allow for the wet and dry phases needed if this silicone gel could act as some sort of cytoplasm?
To begin answering this question, we can look to Caltech where researchers there were able to breed bacteria that could (for the first time ever) form organosilicon compounds (Calvin, 2016) which “Shows how quickly nature can adapt to new challenges" and that "The DNA-encoded catalytic machinery of the cell can rapidly learn to promote new chemical reactions when we provide… the appropriate incentive… Nature could have done this herself if she cared to”. ‘Incentive’, then, in evolutionary terms, could be interpreted as the perhaps a niche an organism could evolve to fill or a challenge (e.g. a change in environmental conditions) that causes an organism to adapt. Either considered, we can conclude silicon must not be very significant for life on an Earth-like planet because not once, in the 3.7 billion years of life on Earth, did the forces of natural selection lead to the incorporation of silicon as an integral feature of any organisms’ survival.
Conclusion
Though silicon, compared to other elements, is the most like carbon and thus the most likely to foster an alternate biochemistry, the likelihood that intelligent life would favour silicon is extremely low considering silicon’s inability to form complex or varied compounds, its tendency to remain inert after reacting, and the unfavourable ‘costs’ these reactions would require. Of course, we may be biased considering carbon-based life is the only one we know of, and even then, we haven’t found such elsewhere, our bias is probably in good stead considering the great variety of life we have here on Earth. In the future, maybe scientists will be able to replicate the formation of biomolecules using silicon entirely in carbon’s place, but if silicon compounds can never come together and replicate themselves without manipulation, life as we define it is unlikely.


 Website By Rejuvenate Digital
Website By Rejuvenate Digital